Abstract
Multiple myeloma (MM) is a malignancy of plasma cells characterized by the production and secretion of monoclonal immunoglobulins. The majority (54%) of MM cases produce an IgG heavy chain containing immunoglobulin, IgA heavy chains are less frequent (22%), but IgA production is associated with worse clinical outcomes. Therefore, we propose that the constant domains of the IgG and IgA heavy chains are promising targets for treatment of patients suffering from MM. In MM, surface immunoglobulin expression is absent, but peptides derived from immunoglobulins will be presented in HLA on the cell surface generating possibilities for T cell receptor (TCR)-based targeting of immunoglobulins.
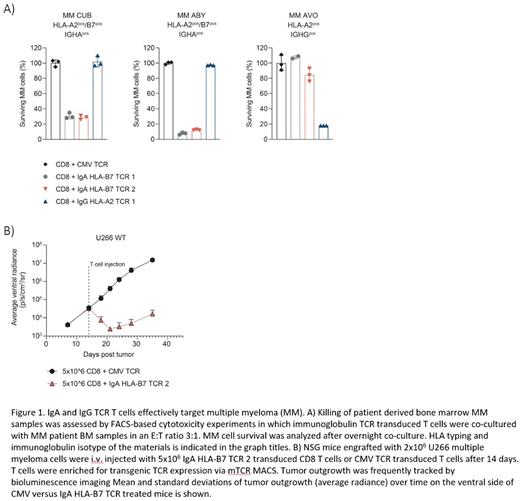
Using a previously established HLA-peptidome database of B-cell malignancies, eleven peptides derived from the IgA or IgG constant domain presented in HLA-A2 or HLA-B7 were identified. For these peptides, peptide-HLA-tetramers were generated and included in ten T cell isolations using complete buffy coats from HLA-mismatched healthy donors. Peptide-HLA-tetramerpos, CD8pos T cells were isolated over an HLA-barrier to allow identification of high affinity T cells specific for these non-mutated peptides. For IgA in HLA-B7, nine T-cell clones recognizing endogenously processed and presented peptide on IgA and HLA-B7 transduced (Td) K562 cells were isolated, from which two T cell clones were selected based on potent recognition of IgApos MM cell line UM6. For IgG, four T-cell clones were identified that recognized IgG and HLA-A2 Td K562 cells of which one T-cell clone recognized IgGpos MM cell line UM3. The three selected IgA and IgG specific T-cell clones demonstrated potent and Ig isotype specific killing of UM3 (IgG) and UM6 (IgA). TCRs of these clones were sequenced and functionality was confirmed by specific tetramer staining and peptide titration experiments after transfer to CD8 T cells. TCR Td CD8 T cells demonstrated comparable functionality to parental T cell clones. In addition, upon co-culture with healthy peripheral blood B cells, IgA and IgG B cells were specifically depleted by IgA and IgG TCR T cells respectively, while healthy cells from non B-cell lineages were not recognized. Furthermore, isotype dependent killing of MM cells in patient derived BM samples was shown and IgA TCR T cells induced significant reduction of MM tumor burden in vivo in a model for established MM using IgAlow U266 cells (Figure 1).
To conclude, we successfully identified TCRs specific for peptides from the constant domains of IgA and IgG that induced potent and specific lysis of Ig isotype positive MM cell lines as well as patient derived MM cells. The IgA HLA-B7 and IgG HLA-A2 specific TCRs identified in this study are promising candidates for further pre-clinical development for TCR gene therapy of patients with MM.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal